CMS released the Medicare Part A Skilled Nursing Facility Prospective Payment System (SNF PPS) FY2022 Proposed Rule this week. The Rule contains the components that will go into effect 10/1/21 pending any changes made based on comments and feedback over the next few months.
The Proposed Rule contains all the “usual,” with updates to Part A rates, ICD-10 code mapping for PDPM, SNF Quality Reporting Program (SNF QRP) changes, and SNF Value Based Purchasing (SNF VBP) updates. The Rule also sneaks in a section that requires specific attention, called: “Recalibrating the PDPM Parity Adjustment.” In this section, CMS shares “some” therapy data and provider practice patterns that knocked PDPM off its’ budget neutral pedestal, and the resulting necessary “recalibration” has the potential to decrease each PDPM daily component rate significantly, depending on the Case Mix Group.
More on that HERE, as it deserves its own article.
In this article, let’s take a quick look at the highlights that are most pertinent to SNF therapy professionals.
Medicare Part A Payment Rate Changes
The Federal Per Diem rates are updated annually. On 10/1/21 the rates for Part A have a proposed increase of 1.3%, which equates to approximately $444 million more in payments to SNFs. These are the unadjusted rates that when multiplied by the case mix index values, determine each PDPM Case Mix Group rate.
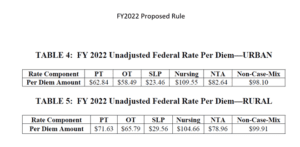
This increase is significantly less than last year’s increase, with the reduction explained in the Proposed Rule, including adjustments and revisions made, as follows. A market basket percentage increase of 2.3 percent was proposed, with a forecast error correction of 0.8%, followed by a 0.2% multi-factor productivity adjustment (MFP), thus resulting in the 1.3% figure. The rule proposed to rebase and revise the SNF market basket index and update the base year from 2014 to 2018. This was last done in the FY2018 SNF PPS Final Rule, which included updating the base year from FY 2010 to 2014.
Medicare Part A PDPM Changes – Case Mix Index Values
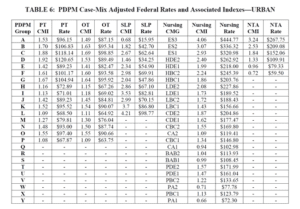
Case Mix Index values for each of the PDPM Components, PT, OT, Speech, Nursing and Non-Therapy Ancillary, are not proposed to change for FY2022 {more on that below…} When multiplied by the Unadjusted Federal Per Diem Rate shown above, Case Mix Group rates will look something like this for FY2022 (Urban). The resulting dollar differences are due to the updates in the Federal Unadjusted Rates above. [See Urban Rates below]
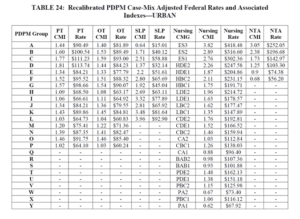
With that said, the “PDPM recalibration” that may {will in some form} take place (see HERE) will impact these numbers significantly, lowering both the Case Mix Index values and rates across the board. The Proposed Rule includes potential of what that may look like with the proposed recalibration method below. PT and OT rates decrease an average of $5 per day each, Speech averages a $3 decrease per day, and Nursing and NTA a $5-26 and $3-15 per day decrease per CMG respectively {and remember, each component is added together to make the total daily rate} {Yikes!} If you compare the recalibrated rates with today’s rates, the recalibrated rates are significantly lower. Depending on the component combinations, the rate could be $10-48/day lower. That’s significant!
Medicare Part A PDPM Changes – ICD-10 Code Mapping for PT, OT and Speech Components
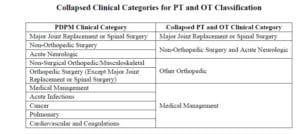
ICD-10 Codes used in MDS Section I0020B as the primary reason for SNF Part A covered care are used for case mix classification under PDPM. Using the Mapping Tool provided by CMS, each ICD-10 Code can be mapped to 1 of 4 PT and OT Clinical Categories as shown here, or identified as a Return to Provider code [meaning this code cannot be used in Section I0020B of the MDS].
Proposed ICD-10 Mapping changes are as follows:
- D57.42 “Sickle-cell thalassemia beta zero without crisis” and D57.44 “Sickle-cell thalassemia beta plus without crisis” currently mapping to Medical Management will map to Return to Provider
- K20.81 “Other esophagitis with bleeding”, K20.91, “Esophagitis, unspecified with bleeding, and K21.01 “Gastro-esophageal reflux disease with esophagitis, with bleeding” currently mapping to Return to Provider will map to Medical Management.
- M35.81 “Multisystem inflammatory syndrome”, currently mapping to Non-Surgical Orthopedic/Musculoskeletal will map to Medical Management
- P91.821 “Neonatal cerebral infarction, right side of brain,” P91.822, “Neonatal cerebral infarction, left side of brain,” and P91.823, “Neonatal cerebral infarction, bilateral,” currently mapping to Return to Provider will map to Acute Neurologic
- U07.0, “Vaping-related disorder,” currently mapping to Return to Provider will map to Pulmonary / Medical Management
- G93.1 “Anoxic brain damage, not elsewhere classified” will change from Return to Provider to Acute Neurologic
Changes In Wage Index – Geographical Locations – For Medicare Part A Calculation
Providers are deemed “urban” or “rural” for purposes of calculating the labor portion of the daily rate, a factor in determining the overall daily rate for a facility. Using wage index values specific to location to adjust the labor portion of the rate is done to reflect the wage differences throughout the country. [Reimbursement rates are different depending on where you live.] There was a significant update in this area effective 10/1/20. The updated Wage Index Tables for FY 2022 can be found below.
Not sure if you are urban or rural? Use the Wage Index Look Up Tool HERE
Changes to Consolidated Billing
The proposed rule includes establishing an additional category of excluded codes, specific to certain blood clotting factors and services for the treatment of hemophilia and other bleeding disorders, from consolidated billing requirements. The specific items and services are those identified by HCPCS codes J7170, J7175, J7177–J7183, J7185–J7190, J7192–J7195, J7198–J7203, J7205, and J7207–J7211. This exclusion impacts the Part A rates as the dollars are shifted from Part A to Part B, and as such, proposed adjustments to the Part A rates from the NTA and Nursing Components of PDPM are included.
The proposed rule also asks for public comments to identify any additional HCPCS codes in the five service categories (chemotherapy items, chemotherapy administration services, radioisotope services, customized prosthetic devices, and blood clotting factors) that might meet criteria for exclusion from SNF consolidated billing.
The latest list of excluded codes can be found on the SNF Consolidated Billing website at https://www.cms.gov/Medicare/Billing/SNFConsolidatedBilling.
Changes to CMS Medicare Part A Quality Programs
SNF Quality Reporting Program (SNF QRP)
- The SNF QRP is the program behind Section GG, as well as other MDS data collection to measure multiple areas of care. Think skin….falls….function. Data collection for the multiple measures in this program will continue, and facilities will continue to be subject to a 2% Part A payment penalty for submission of Part A MDS’s with missing (dashed) data in select items.
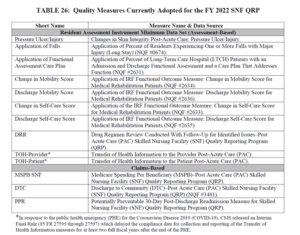
- Here are the 13 Measures in place for FY2022:
- CMS proposes to adopt two new measures for the SNF QRP beginning with the FY 2023:
- SNF Healthcare-Associated Infections Requiring Hospitalization measure (SNF HAI)
- This an outcome measure, and per CMS, “the data would allow providers to exchange and compare outcomes across the care continuum and Post Acute Care (PAC) settings.” “Clinical data captured in every clinical setting informs a resident’s current care plan, facilitates coordinated care, and improves Medicare beneficiary outcomes,” and CMS plans to develop HAI measures in other PAC settings, such as the Inpatient Rehabilitation Facility (IRF) Quality Reporting Program and the Long-Term Care Hospital (LTCH) Quality Reporting Program.
- COVID-19 Vaccination Coverage among Healthcare Personnel (HCP)
- CMS identified this measure as a priority in response to the current public health crisis. This process measure was developed with the Centers for Disease Control and Prevention (CDC) to track COVID-19 vaccination coverage among HCP in the SNF setting.
- CMS states the importance of requiring SNFs to report staff vaccinations in order to assess whether they are taking steps to limit the spread of COVID-19 among their workers, reduce the risk of transmission of COVID-19 within their facilities, and to help sustain the ability of SNFs to continue serving their communities throughout the PHE and beyond.
- Under the May 8, 2020 Interim final rule, SNFs are required to submit COVID-19 data through the CDC’s NHSN Long-term Care Facility COVID-19 Module of the NHSN. Examples of data reported in the module include: suspected and confirmed COVID-19 infections among residents and staff, including residents previously treated for COVID-19; total deaths and COVID-19 deaths among residents and staff; personal protective equipment and hand hygiene supplies in the facility; ventilator capacity and supplies available in the facility; resident beds and census; access to COVID-19 testing while the resident is in the facility; and staffing shortages. Although HCP and resident COVID-19 vaccination data reporting modules are currently available through the NHSN, the reporting of this data is voluntary.
- CMS states they believe that publishing facility-level COVID-19 HCP vaccination rates on Care Compare would be helpful to many patients, including those who are at high-risk for developing serious complications from COVID-19, as they choose facilities from which to seek treatment.
- SNF Healthcare-Associated Infections Requiring Hospitalization measure (SNF HAI)
SNF Value-Based Purchasing Program (SNF VBP)
- The SNF VBP Program is the one that reduces Medicare Part A payments to all SNFs by 2% and then allows for an opportunity to recoup all or part of the 2% by demonstrating success with the established Quality Measure, SNF 30-Day-All-Cause Readmission Measure. In essence, this measures if the resident was readmitted back to the hospital within 30 days of being discharged from the hospital. The proposed rule includes the Achievement Threshold and Benchmark data for FY2024 as pictured here, though no significant changes were made to the program for FY2022 that will impact SNF therapy.

- There are, however, changes that will impact the SNF and payment. CMS is proposing to adopt a policy for the duration of the PHE for COVID-19 that would suppress the use of SNF readmission measure data for purposes of scoring and payment adjustments in the SNF VBP Program. CMS states this “is a necessity to ensure that the SNF VBP program does not reward or penalize facilities based on factors that the SNF readmission measure was not designed to accommodate.” CMS intends for this proposed policy to provide short-term relief to SNFs.
- Under this proposed suppression policy, for all SNFs participating in the FY 2022 SNF VBP program, CMS would use the previously finalized performance period and baseline period to calculate each SNF’s risk-standardized readmission rate for the SNFRM, followed by assigning all SNFs a performance score of zero, resulting in identical performance scores and identical incentive payment multipliers.
- CMS would provide each SNF with its SNF readmission measure rate in confidential feedback reports so that the SNF is aware of the observed changes to its measure rates, and rates would be publicly reported with appropriate caveats noting the limitations of the data due to the PHE for COVID-19.
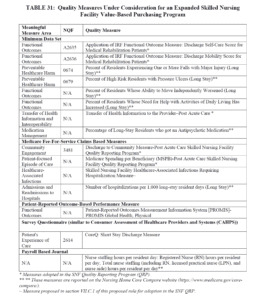
- CMS also outlines consideration for additional VBP measures, including: functional status, patient safety, and care coordination, as well as including measures expanded to all residents, not only Part A, and is requesting public comment. The list of potential measures is below:
In Summary
We have new proposed rules to digest, as well as keeping step with the current public health emergency and the temporary rules associated with it. Waivers are still in place and are expected to last through 2021. You can read more about that HERE.
CMS is accepting comments on this proposed rule through June 7, 2021.
HERE is a link to the Proposed Rule. [PDF Copy HERE]
HERE is a link to the CMS Fact Sheet.
If you have any questions, send them to our Just Ask Q&A Forum and we will reach out to you to get your questions answered.
In your corner,
Dolores
Dolores Montero, PT, DPT, RAC-CT, RAC-CTA


Delores,
I have a question regarding when a patient with a Managed Medicare A benefit is denied and the patient or family wants to appeal. I know we must continue to provide services thru the appeal but does the liability lie with the beneficiary if they loose the appeal or is it okay to start MCB services on the last day of coverage and bill accordingly?
Hi Brian – Thank you for the question! I am sending this to JustAsk Q&A Forum and a team member will respond to you via email. Thank you!